





Welcome
Welcome

Mechanisms of stem cell aging and transformation
Hematopoietic stem cells (HSC) maintain multi-lineage blood formation throughout our lifetime. Balancing stem cell regeneration and differentiation commitment to produce mature blood cells is quintessential for a healthy hematopoietic system. Dysregulation of such HSC fate determination processes can lead to loss of immune function, bone marrow failure, and malignant transformation during aging. However, up to date very little is known about the molecular events driving age-related HSC changes and how they contribute to disease.
Understanding age-associated molecular alterations will not only uncover fundamental mechanisms guiding function of HSCs, but may also allow for therapeutic intervention to “rejuvenate” aged hematopoietic systems and possibly even prevent age-associated hematopoietic diseases. Our mission is to clarify the central mechanisms establishing and guarding sustained hematopoietic stem cell function, particular those that drive leukemogenesis, if disrupted.
We develop innovative genetic mouse models, use ex vivo and in vivo primary mouse and human stem cell assay systems, exploit lentiviral gene transfer, and apply state-of-the-art molecular biology and next generation sequencing techniques.
The labile iron pool as a rheostat for stem cell function
Our recent work has uncovered a key role of the amount of readily accessible intracellular iron (termed labile iron pool, LIP) in instructing HSC self-renewal. We are currently investigating the precise molecular mechanism of action, particularly focusing on metabolic and non-enzymatic molecular pathways relying on iron – a completely uncharted territory for healthy as well as leukemic stem cells.
Gene expression program erosion in aging stem cells and leukemia
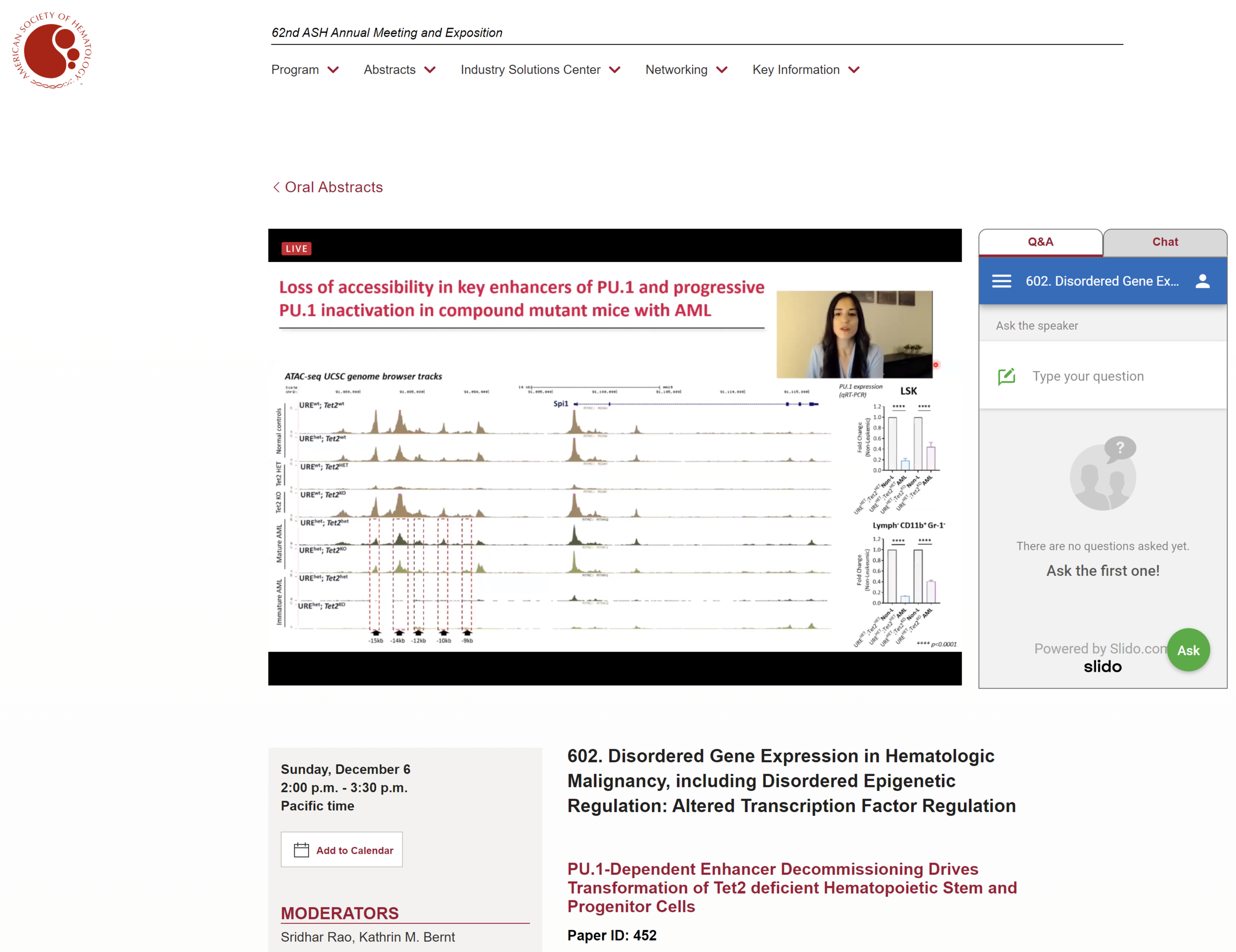
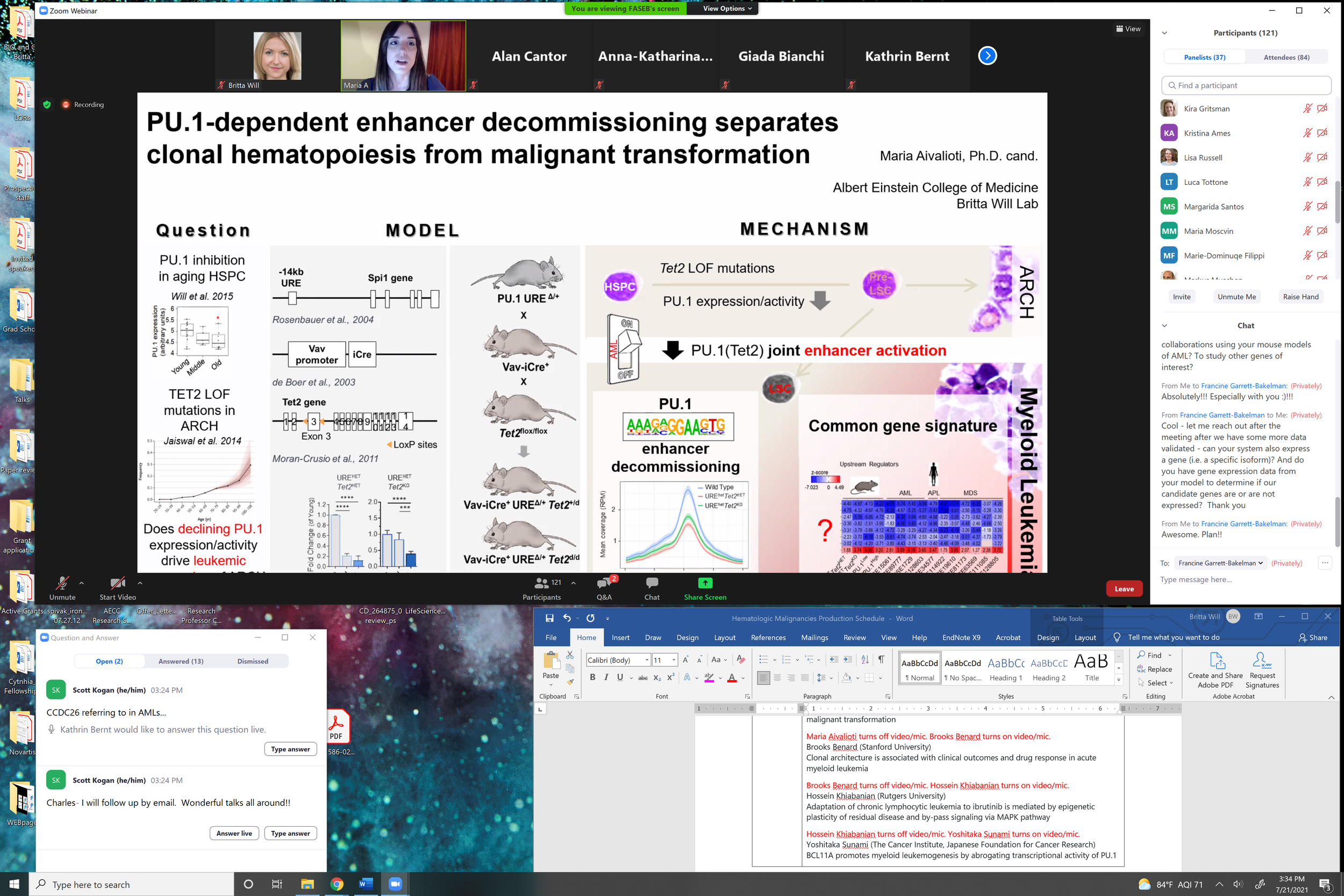
Our past work has demonstrated a causative role of even minimal dosage alterations of a key transcription factor instructing hematopoiesis, PU.1, observed in hematopoietic stem cell aging to myeloid leukemia evolution. Our current efforts focus on understanding how such slight deviations from optimal PU.1 dosage lead to the erosion of PU.1-dependent gene expression programs, and in which way a slightly altered PU.1 gene expression network can functionally cooperate with age-associated inactivation of epigenetic regulators (TET2 and DNMT3A).
Identification of molecular safeguards of cancer stem cells
Teamed-up with Dr. Ana Maria Cuervo (Dept. of Developmental & Molecular Biology), the discoverer of a highly precise protein degradation pathway named chaperone-mediated autophagy, we are investigating the role of this stress-related molecular defense mechanism in leukemic stem cell evolution and maintenance.

Principal Investigator
Principal Investigator
Britta Will, Ph.D.
Director, The Ruth L. and David S. Gottesman Institute for Stem Cell Research and Regenerative Medicine
Director, Preclinical Modeling, Montefiore Einstein Comprehensive Cancer Center Blood Cancer Institute
Co-Leader, Montefiore Einstein Comprehensive Cancer Center, Stem Cell & Cancer Biology Program
Associate Professor,
Department of Oncology (Medical Oncology)
Department of Medicine (Oncology & Hematology)
Ruth L. and David S. Gottesman Chair in Stem Cell and Regenerative Medicine Research

MEMBERS
MEMBERS
Our Team
Britta Will, PhD
Principal Investigator
Director, Gottesman Institute for Stem Cell Biology and Regenerative Medicine.
Associate Professor, Cell biology, Oncology (Medical Oncology), Medicine (Oncology & Hematology)
Malini Gupta, PhD
Staff Scientist
I moved to USC Columbia from Calcutta, India to pursue my PhD in Chemistry before starting my postdoctoral stint in the Will Lab in 2020 at Albert Einstein. I am currently a Staff Scientist working at the nexus of iron homeostasis and leukemia.
Outside the lab, landscape and street photography serve as my creative escape, giving me an excuse to wander with my camera—whether exploring the city or seeking quieter spots away from the crowd. To unwind, I prefer settling in with some good cinema and a comforting cup of tea.
Mehrnaz Safaee Talkhoncheh, PhD
Postdoctoral Researcher
Bio Incoming
Victor J. Thiruthuvanathan, M.Pharm.
Staff Scientist
After 6 years as a Quality Control Scientist in India’s biotech industry, I moved to the U.S. to pursue biomedical research. Since 2016, I have worked in this lab, specializing in cancer cell biology, with a focus on preclinical modeling and the discovery of novel therapeutic agents for AML and MDS.
I love playing music and often unwind by taking long drives to remote places— my favorite way to recharge and find inspiration
Xiangshang Li (Kevin), PhD
Computational Biologist
I hold a PhD in computational biology. My research focuses on decoding the molecular regulation of acute myeloid leukemia (AML), with a special interest in iron homeostasis and chaperone-mediated autophagy (CMA). By integrating computational modelling and genomic insight, I aim to identify novel regulatory nodes that can serve as translational targets and ultimately improve outcomes for patients with AML.
Dror Perk, MS
MD/PhD Student
I am focused on understanding the role of the transcription factor PU.1 in human hematopoiesis and how it contributes to leukemic transformation. Towards this goal I have established a novel in vitro model in which the PU.1 gene has been degron-tagged and can be abltated at will within human iPSC cells.
For fun I love woodworking, playing chess, and occasionally playing the guitar.
Madison Smith
MD/PhD Student
I just joined the lab in April of 2025. Here I am studying iron metabolism, dormancy, and differentiation in colorectal cancer.
In my free time, I enjoy exploring the city, hiking, reading, and cooking.
PAST MEMBERS
CORE TEAM:
Yuhong Ma, PhD - Postdoctoral Scientist
Yun-Ruei Kao, PhD - Research Assistant Professor
Maria Aivalioti, M.Sc. - PhD Student
Aliona Zintiridou - Research Technician
Elizabeth Pan, M.Sc. - PhD Student
Madhuri R. Tatiparthy, M.Sc. - Research Technician
Sonia Gallego, B.Sc. - Lab Manager/Research Technician
Mariana Ferreira, M.Sc. - Research Technician (2015-2018), now research technician at MSKCC
Margarida Teixeira, Ph.D. - Postdoctoral Research Fellow (2016-2018), now Research Applications Scientist with Beckton Dickinson, NY, NY
Lumie Benard, M.Sc. - Research Technician CSC-PD (2015-2019), now Research Technician at Stelexis Pharmaceuticals, Bronx, NY
Sally Cole, B.Sc. - Research Technician CSC-PD (2016-2018), now Ph.D. student at Columbia University, NY, NY
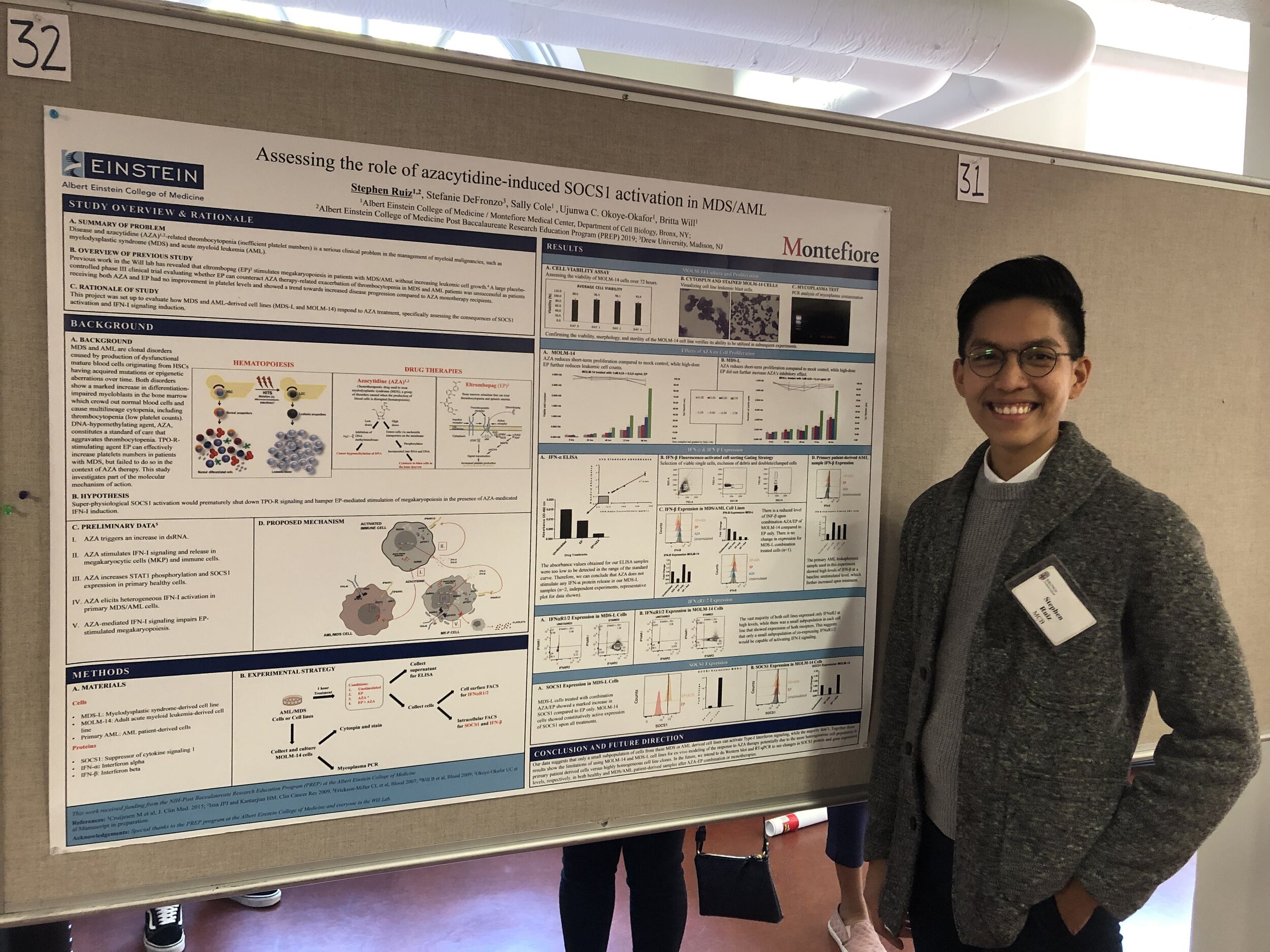
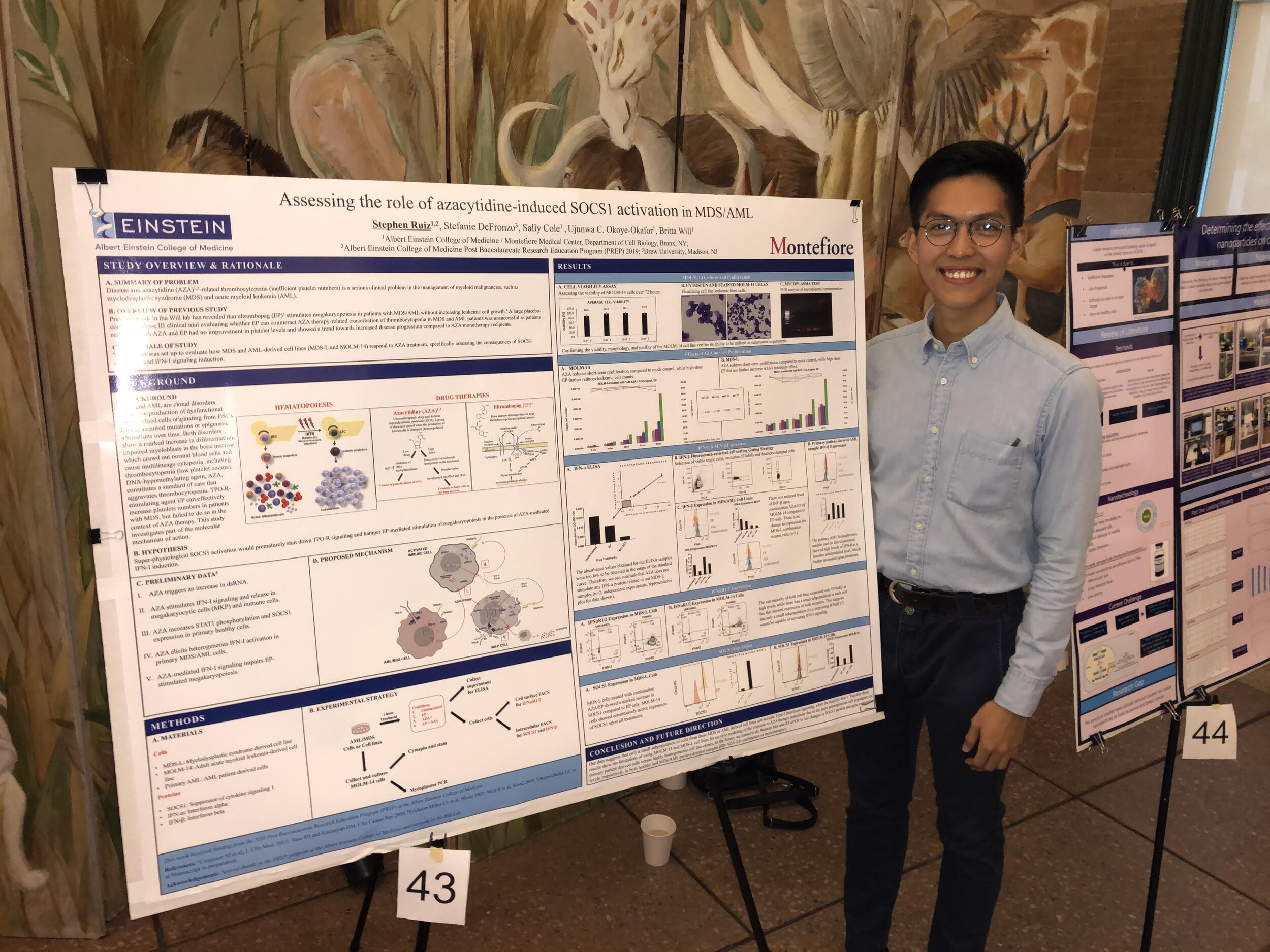
Stephen Ruiz, B.Sc. - Research Technician and Web Master (2017-2019), now Ph.D. student at Weill Cornell Medical Center, NY, NY
Ujunwa C. Okoye-Okafor, Ph.D. - Postdoctoral Fellow and Research Associate (2018-2021), now Senior Research Associate at Hackensack Meridian Health
lab events














































PUBLICATIONS
PUBLICATIONS
Chaperone-mediated autophagy sustains hematopoietic stem-cell function
Spearheaded by Dr. Shuxian Dong and in collaboration with Dr. Ana Maria Cuervo, we studied the role of chaperone-mediated autophagy (CMA), a selective form of lysosomal protein degradation, in sustaining HSC function in adult mice. We found that CMA is required for protein quality control in stem cells and for the upregulation of fatty acid metabolism upon HSC activation. We uncovered that CMA activity decreases in HSC with age and show that genetic or pharmacological activation of CMA can restore the functionality of old mouse and human HSCs. Together, our findings provide mechanistic insights into a role for CMA in sustaining quality control, appropriate energetics and overall long-term HSC function. Our work suggests that CMA may be a promising therapeutic target for enhancing HSC function in conditions such as ageing or stem-cell transplantation. (Dong et al., Nature 2021)
Comment by Drs. Nick van Gastel and David T. Scadden in Cell Research
Thrombopoietin receptor–independent stimulation of hematopoietic stem cells by eltrombopag
In this new study, we characterized the mechansim of action of eltrombopag on stimulating hematopoietic stem cell function and uncovered a key role of the amount of readily accessible intracellular iron in instructing hematopoietic stem cell self-renewal (Kao et al., STM 2018).
Minimal PU.1 reduction induces a preleukemic state and promotes development of acute myeloid leukemia
Hematopoietic stem cell aging is associated with wide-ranging gene expression alterations. Here we demonstrated that even minimal dose alterations of a key hematopoietic transcription factor, PU.1, predisposes stem cells to malignant transformation (Will et al., Nat. Med. 2015).
Satb1 regulates the self-renewal of hematopoietic stem cells by promoting quiescence and repressing differentiation commitment
Here, we identified the transcription factor and chromatin remodeler Satb1 as a critical regulator of HSC fate, and provide insight into how hematopoietic stem cells coordinate the regulation of opposing cellular mechanisms such as self-renewal and differentiation commitment upon cell division (Will et al., Nat Immunol 2013).
Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations
Myelodysplastic syndrome (MDS) is an ageing-associated hematologic malignancy, largely lacking curative therapies. In this study we demonstrated that patient-derived aberrant stem and progenitor cells contain epigenetic and genetic alterations which may be exploited for therapeutic targeting (Will et al., Blood 2012).
Effect of the nonpeptide thrombopoietin receptor agonist Eltrombopag on bone marrow cells from patients with acute myeloid leukemia and myelodysplastic syndrome
Thrombocytopenia is a frequent symptom and clinical challenge in patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Eltrombopag is a small molecule thrombopoietin receptor agonist that might be a new option to treat thrombocytopenia in these diseases, provided that it does not stimulate malignant hematopoiesis. In this work, we provide a preclinical rationale for further testing of Eltrombopag for treatment of thrombocytopenia in AML and MDS (Will et al., Blood 2010).
For a full list of publications, please click here

OPEN POSITIONS & CONTACT
OPEN POSITIONS & CONTACT
Open Positions
We are always looking for passionate scientists at all experience levels to join our team. If you are interested in working with us, please send your application package consisting of your current resume or CV including the contact information of at least two recent professional references and a cover letter briefly describing your background and your motivation.
Contact Us
Britta Will, Ph.D. Telephone Office : +1 (718) 430-3786
Albert Einstein College of Medicine Main Lab : +1 (718) 430-6430 or 6461
Department of Cell Biology
Chanin Building for Cancer Research Rooms 401/401A and 302B
1300 Morris Park Avenue
Bronx, NY 10461
USA

















